The Science-Fair Projects of Allen Bukoff
Cloud Chamber for Studying Nuclear Particles
Coon Rapids High School Science Fair
and The State Science Fair of Iowa
Spring, 1966
Fourteen photos of the tracks left by nuclear particles.
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
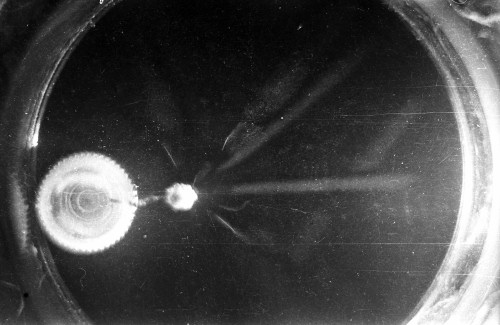
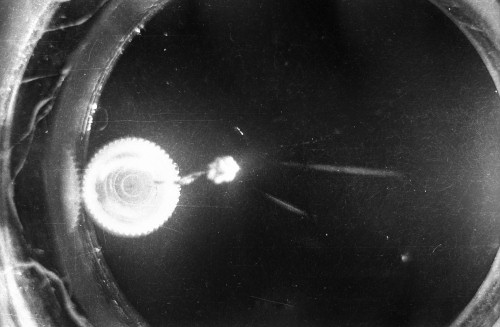
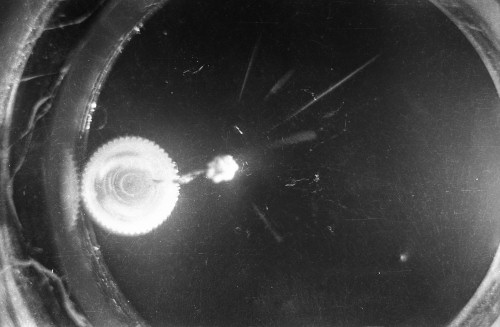
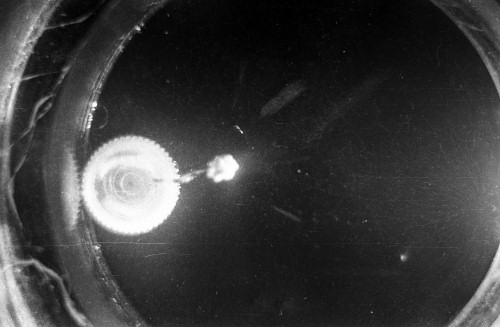
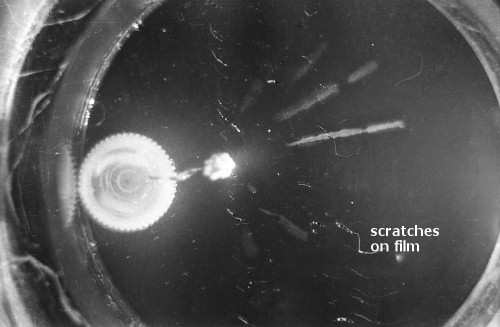
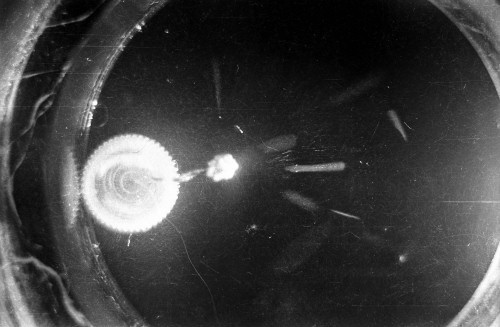
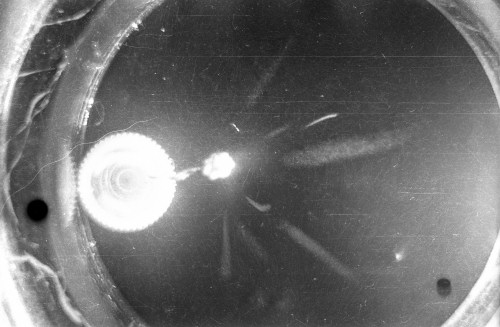
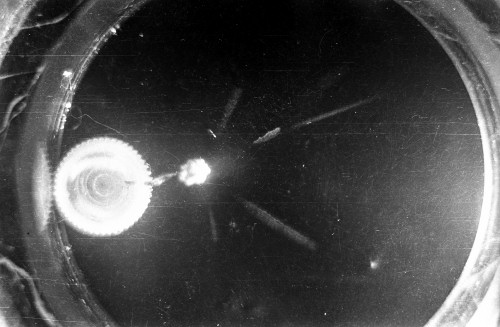
I constructed two different cloud chambers for one of my first science fair projects (I was a freshman in high school in 1966). One of the cloud chambers used dry ice to cool evaporating isopropyl alcohol to form a supersaturated mist in which the charged nuclear particles would create a temporary, visible vapor trail.; The visible vapor trail is formed when some of the floating alcohol molecules are ionized/charged as the particle moves across the cloud chamber. These ionized alcohol molecules then attract enough other floating alcohol molecules to form a visible white drop--a whole string of white drops--as the particle moves across the cloud chamber. These trails or tracks only last a moment before they fall like tiny rain drops to the bottom of the cloud chamber. The other type of cloud chamber I constructed for this project--an expansion-type cloud chamber--used the partial vacuum created by a bathroom plunger to lower the pressure (and temperature) within the chamber to create a mist of isopropyl alcohol without the use of dry ice.
The black-and-white photos shown above were taken using a 35mm camera positioned over the dry ice cloud chamber. A small ball of paint containing radium was used as the source for particles. The radium paint was scraped from old glow-in-the-dark watch faces and glued into a small ball on the end of a wire that was then stuck in a small plastic cap. This project demonstrated how cloud chambers functioned and how physicists had used them to study subatomic particles. The sharp white lines in the photos above were created by alpha particles (two protons and two neutrons--essentially the positively charged nucleus of a helium atom). The fuzzier, softer white lines were created by beta particles negatively-charged free electrons. A beta particle is weaker in charge and mass than the alpha particle--hence the weaker, fuzzier trail it leaves in the mist. Other types of particles and rays leave other distinctive patterns or tracks. By varying the source of the radioactivity, and by using magnets, electrically charged fields and other interventions, early twentieth century physicists were able to use cloud chambers to identify and explore a variety of new subatomic particles, waves, and properties. C.T.R. Wilson was awarded the Nobel Prize in Physics in 1927, for inventing the cloud chamber and for recognizing its use for studying ionization. Another physicist of that era, Ernest Rutherford, described the cloud chamber as "the most original and wonderful instrument in scientific history." Instructions for making your own homemade cloud chamber can be found here or here. You can also buy commercially manufactured cloud chambers here or here. |
 |
| © 2007 |